Question Video: Identifying Which Metal Can React with Acid and Its Oxide Can Be Reduced by Carbon | Nagwa

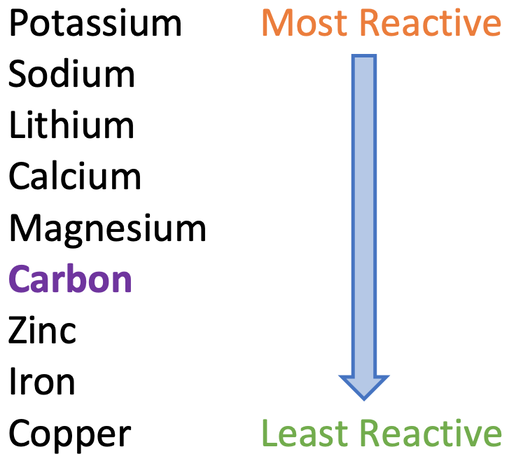
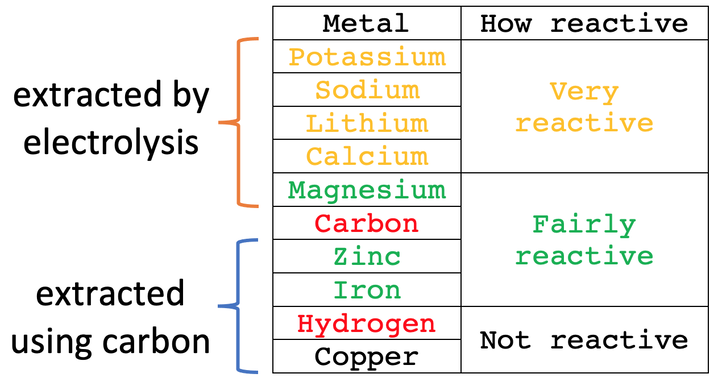
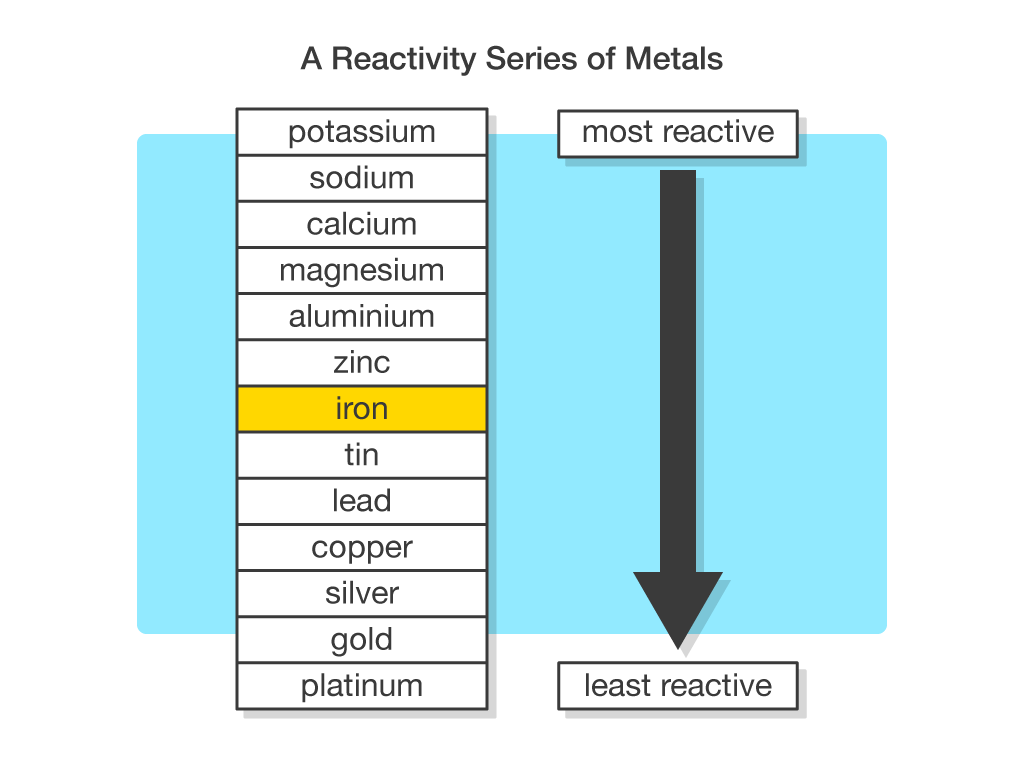
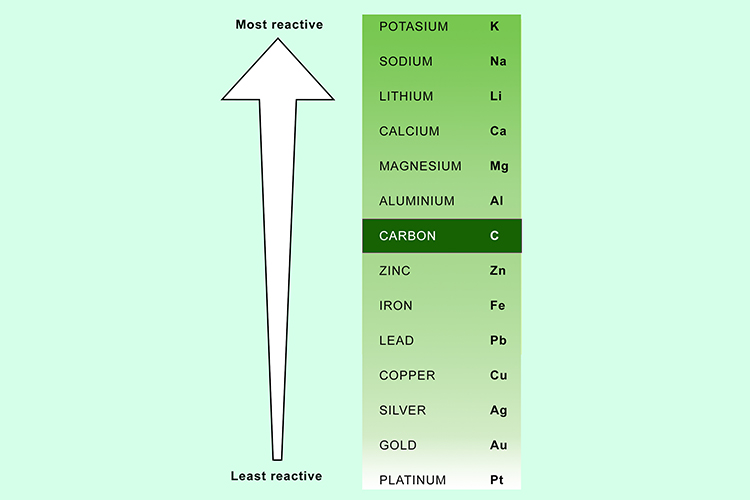
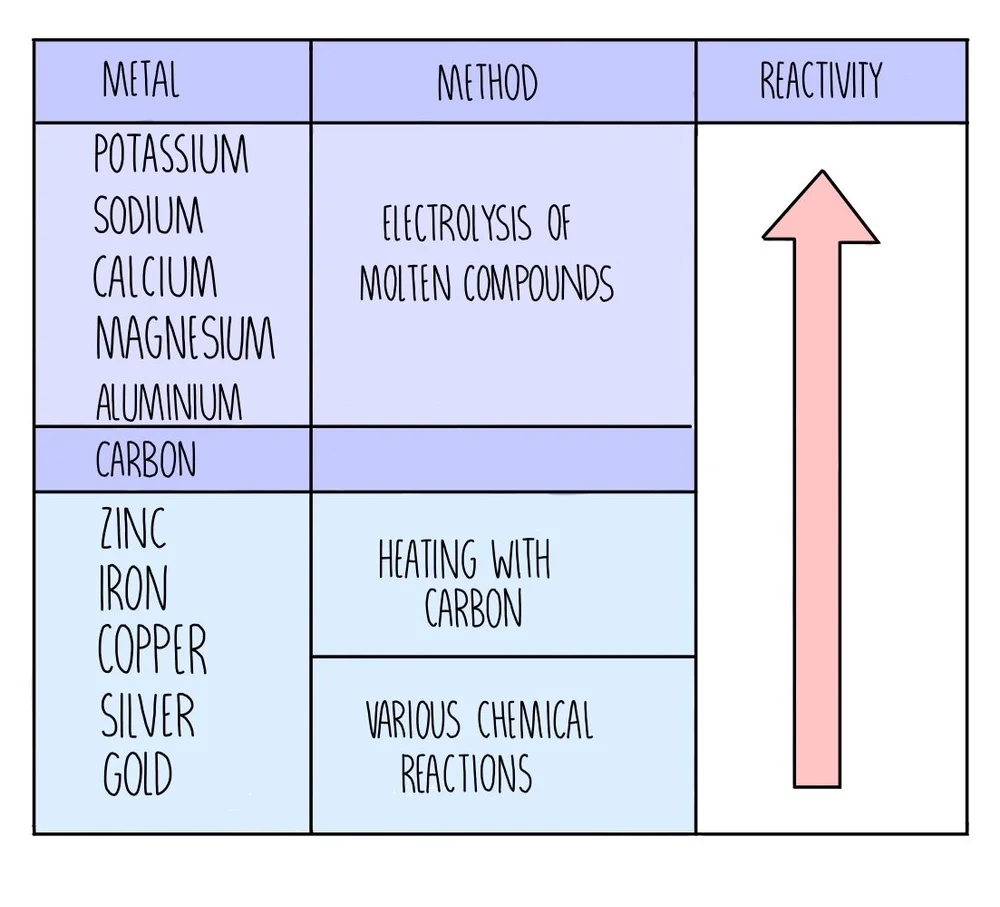
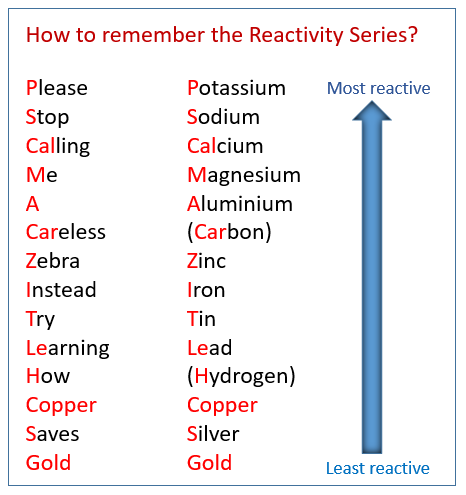
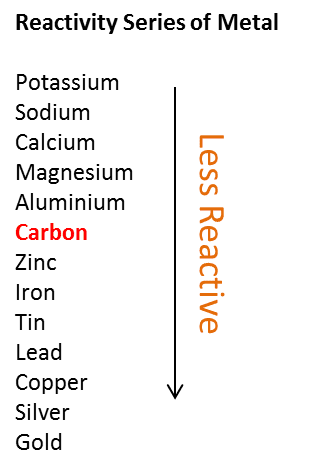
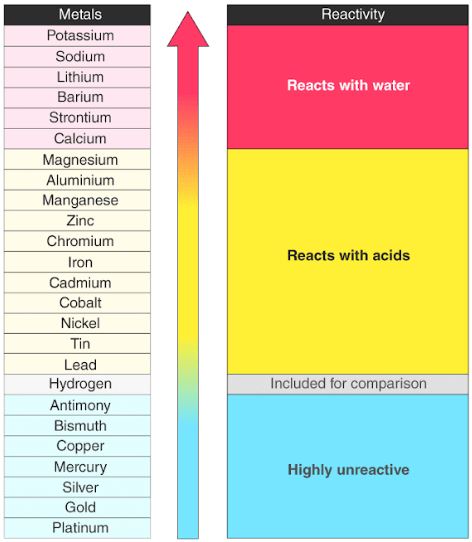
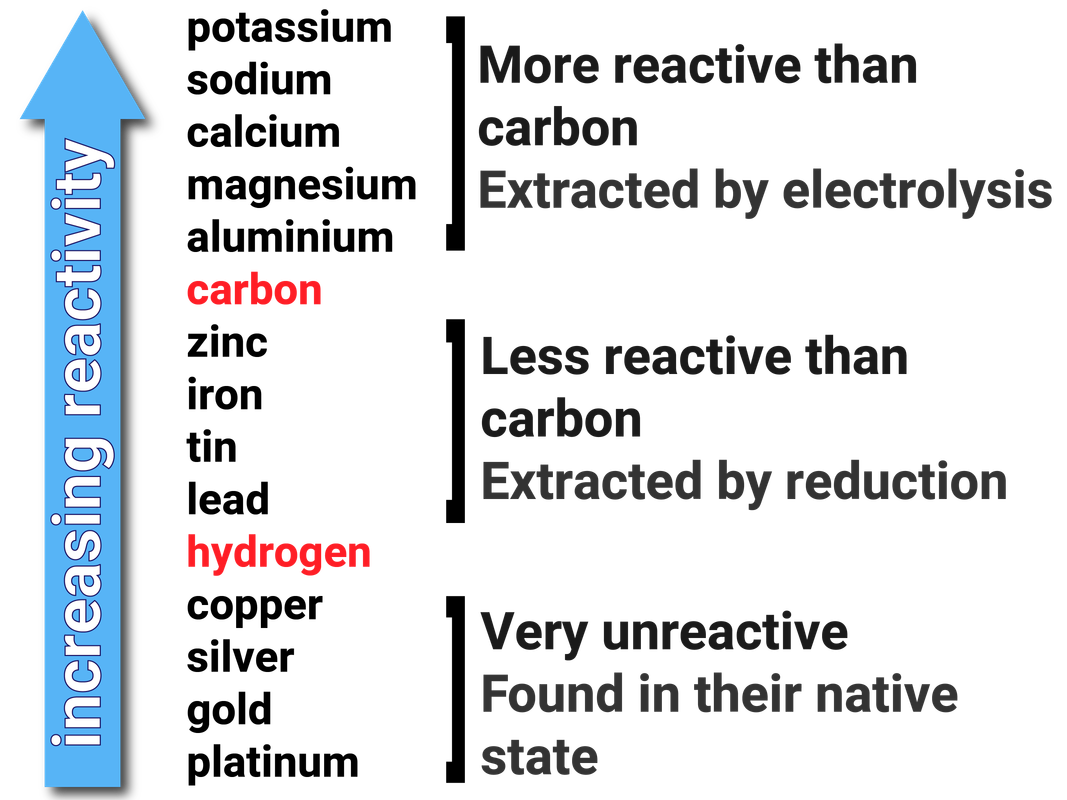
Extraction of Iron. As we have already discussed, carbon can be used to extract any metal found below it in the reactivity series. Potassium Sodium Magnesium. - ppt download
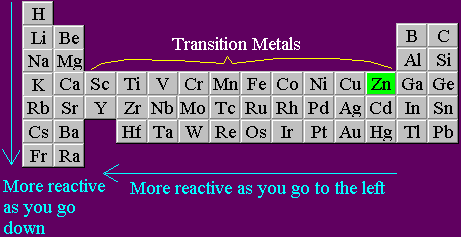
In the reactivity series, what makes iron more reactive than copper? In chemistry, what is reactivity? Why are some elements/compounds more reactive than others? - Quora