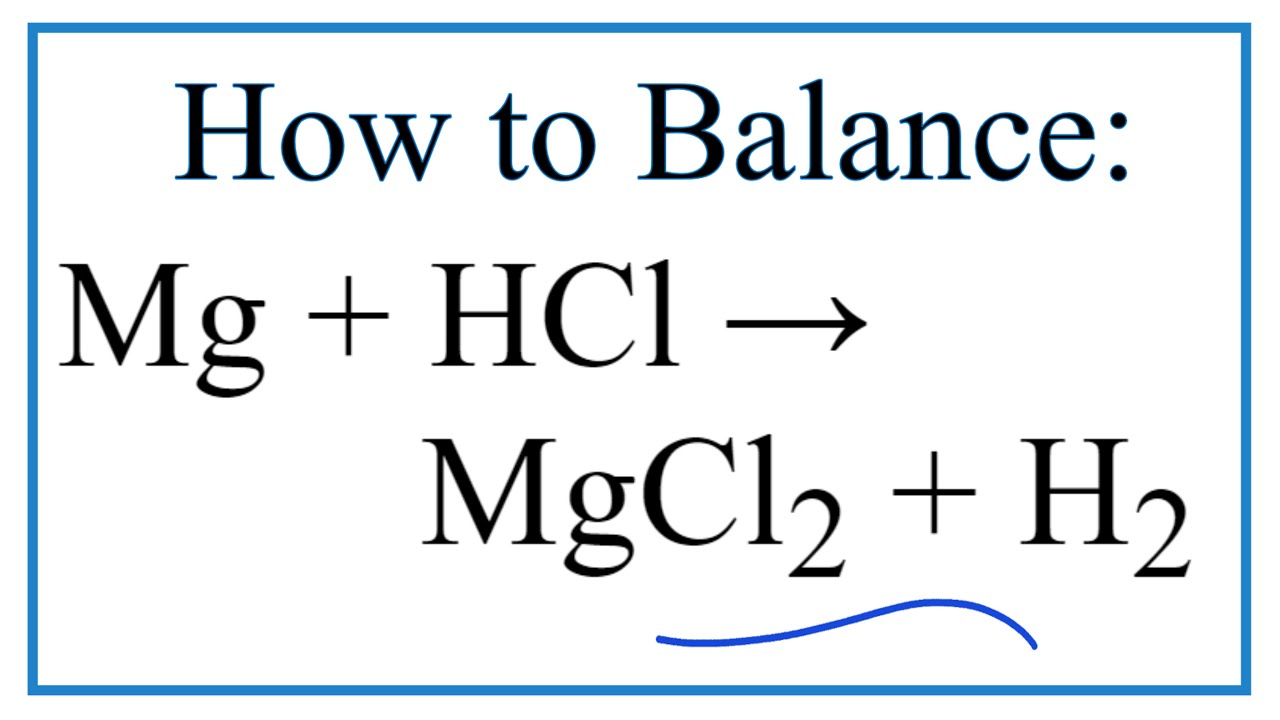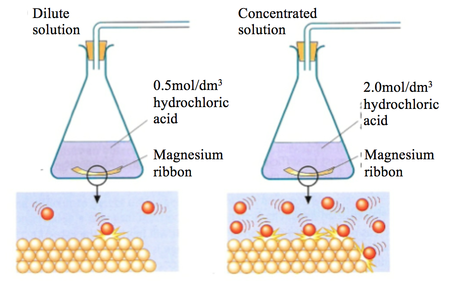
4.20 explain the effects of changes in concentration of solutions and pressure of gases on the rate of a reaction in terms of particle collision theory - iGCSE CHEMISTRY REVISION HELP

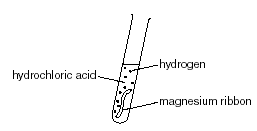
Magnesium Reacts With Dilute Hydrochloric Acid in A Conical Flask Which Is Connected To An Inverted Measuring Cylinder in A Trough of Water | PDF

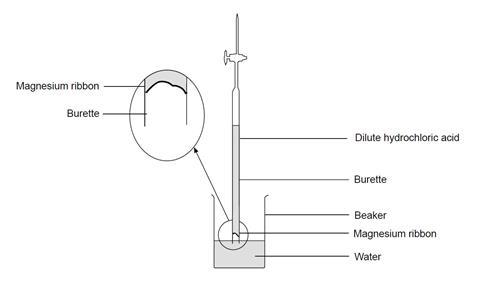
Describe an experiment to study the speed of reaction between dilute hydrochloric acid and magnesium, by measuring the volume of gas produced over time. - Study notes, tips, worksheets, exam papers