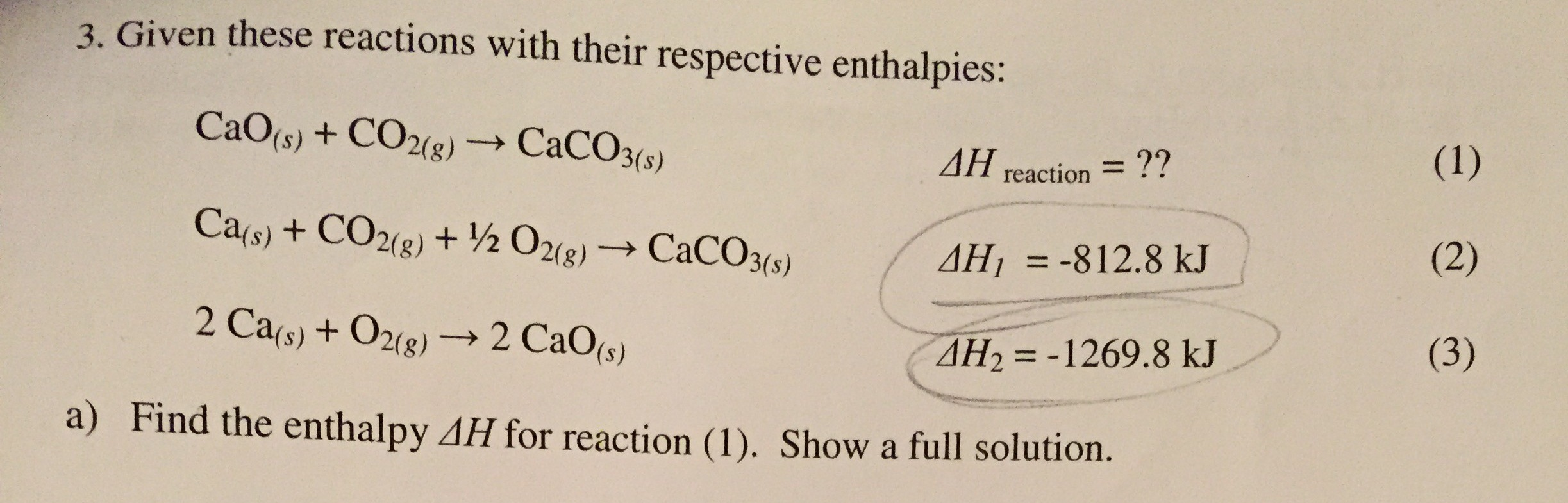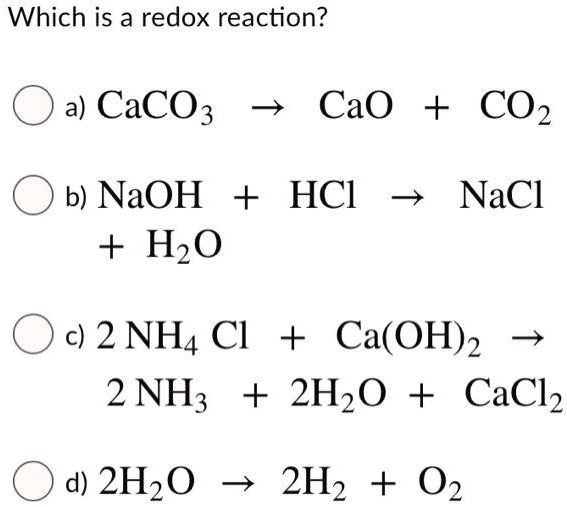
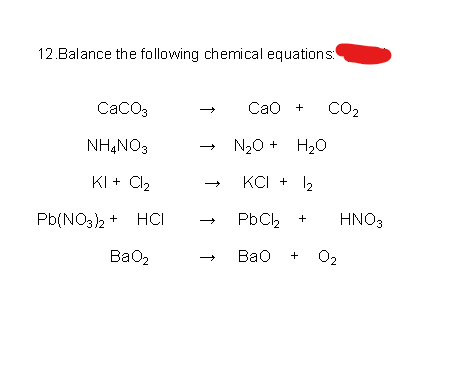
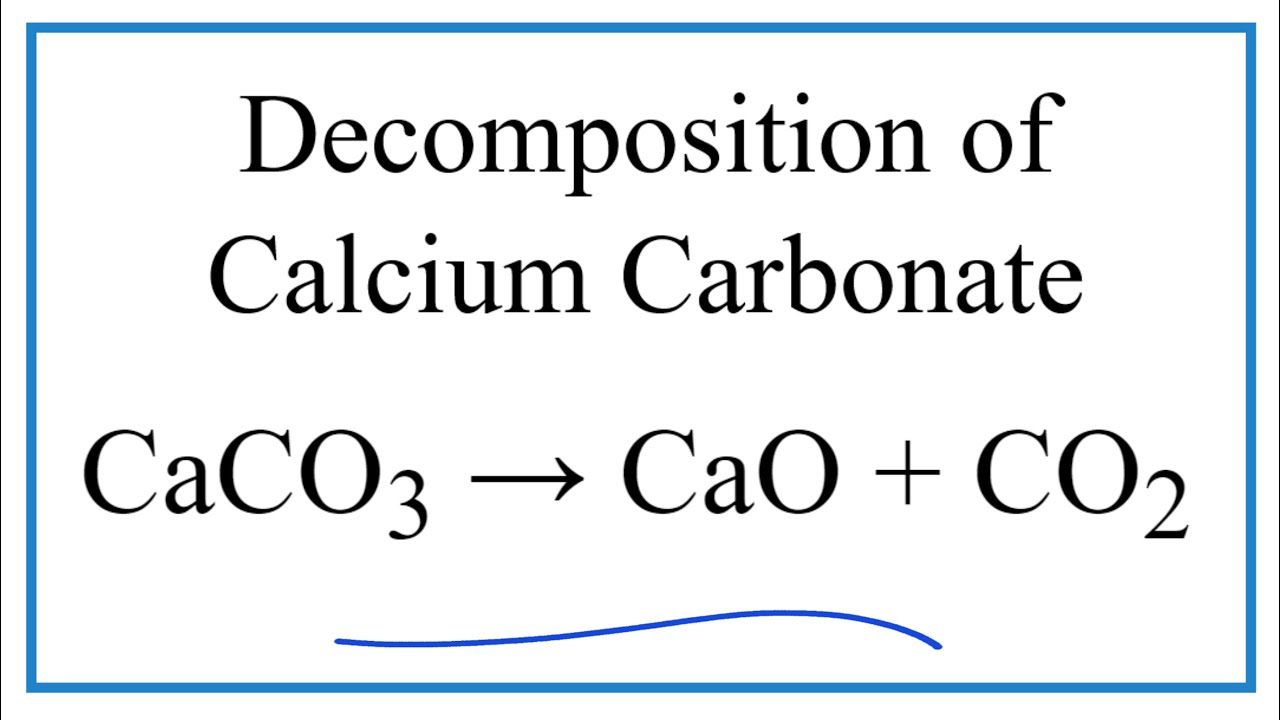
SOLVED: Which is a redox reaction? a) CaCO3 CaO + CO2 b) NaOH + HzO HCl NaCl c) 2 NH4 Cl + Ca(OH)2 2 NH; + 2Hz0 + CaClz d) 2Hz0 2H2 + 02
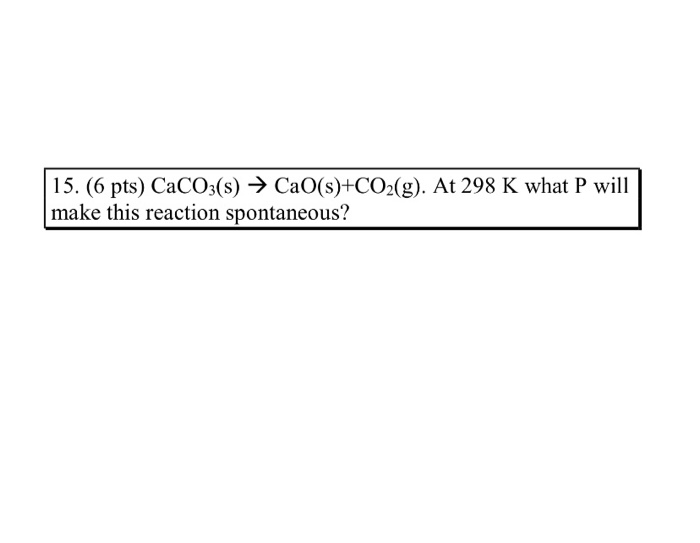
In the equilibrium CaCO3(s)Cao (s)+CO2(g) at 1073 K, the pressure of CO2 isfound to be 2.5 x 10 Pa. The equilibriumconstant for the reaction at 1073 K will be.(a) 0.25(c)25(b) 2.5(d) 250

CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions - ScienceDirect
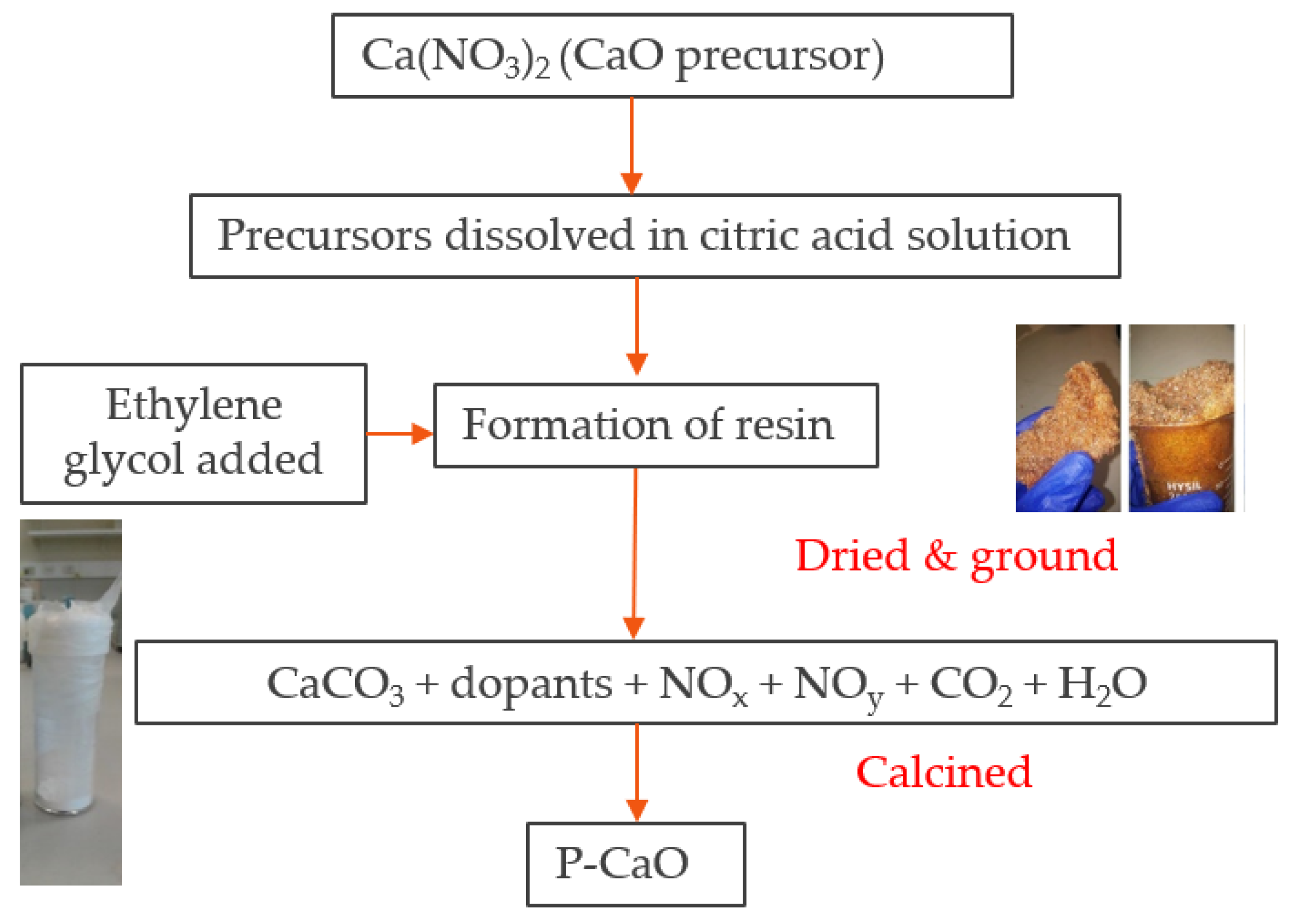
Applied Sciences | Free Full-Text | Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture
CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system - RSC Advances (RSC Publishing)
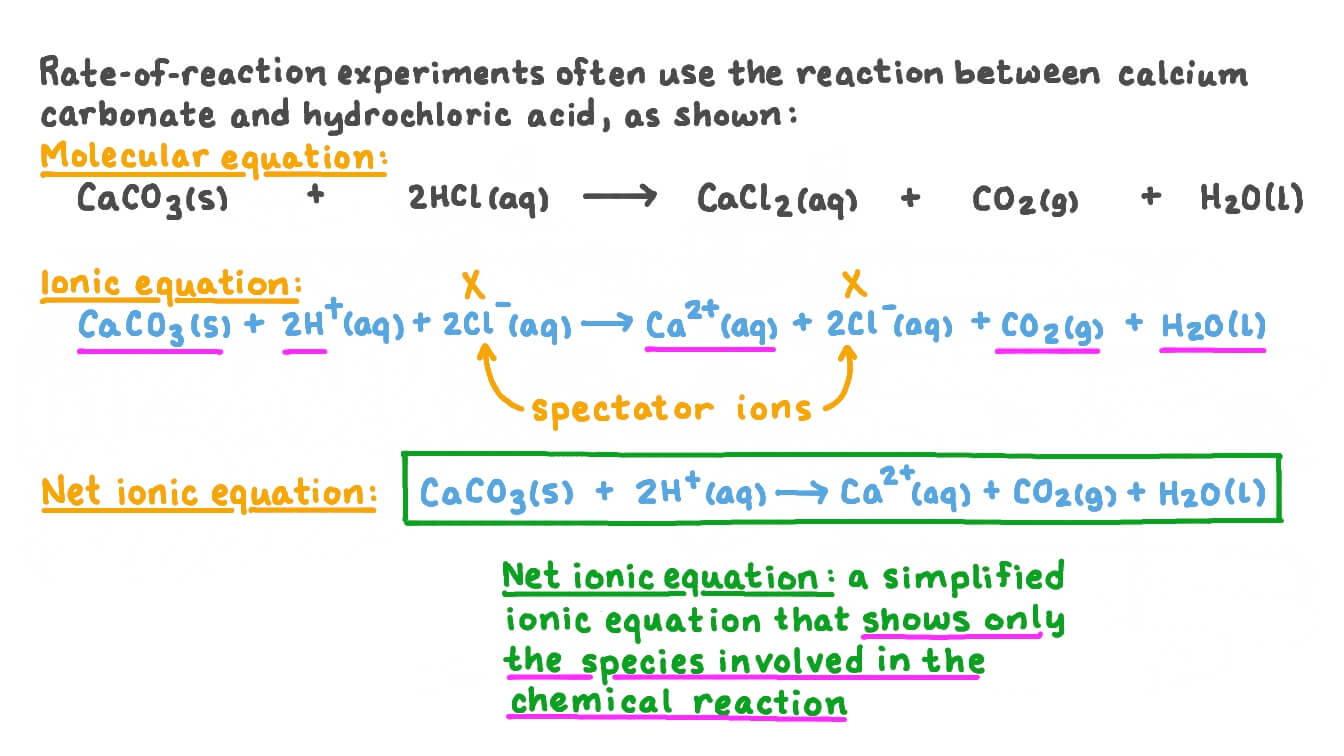
Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
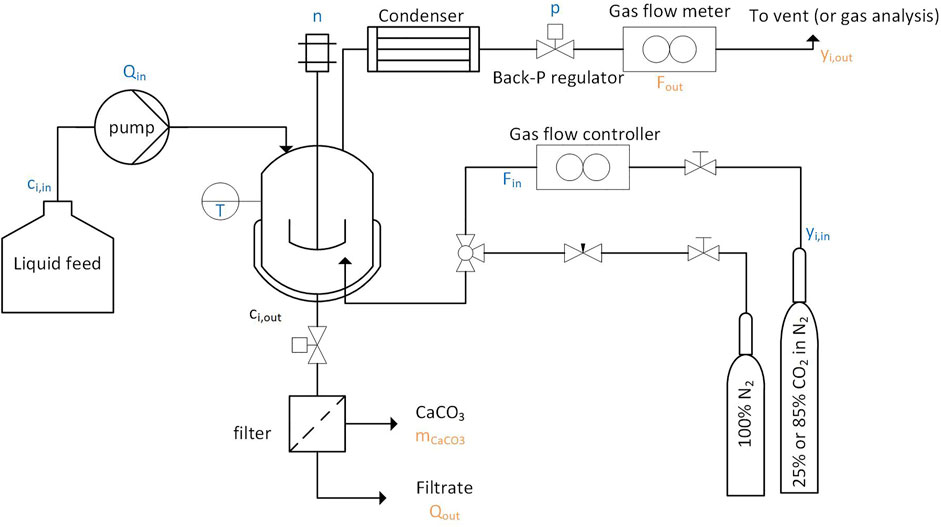
Frontiers | Experimental Investigation of a Continuous Reactor for CO2 Capture and CaCO3 Precipitation
![CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is](https://dwes9vv9u0550.cloudfront.net/images/2234632/d247a5dd-b799-4377-806f-2628447e070f.jpg)
CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is

SOLVED: 1 1 point Use Hess's Law to calculate AHO for the reaction: CaO + CO2 CaCO3, given: Ca + CO2 + Yz 02 1 CaCO3 AHO =-813 kJ 2 Ca +

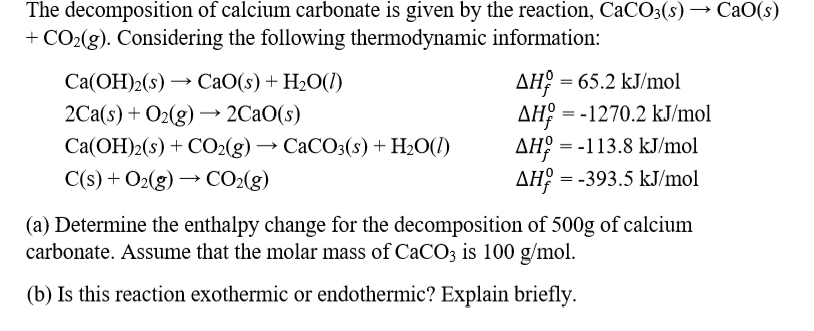


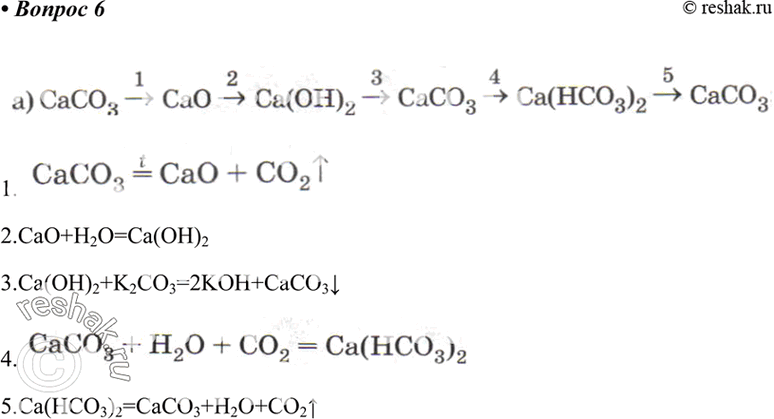


![PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/70ca22dce93c5e0c4e267a02ccc4e41951e44132/2-Figure2-1.png)