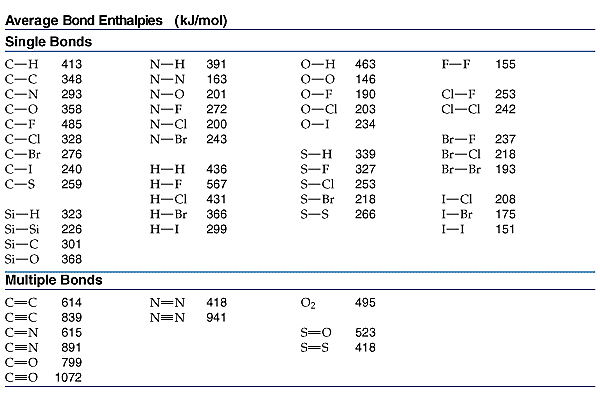
12 Methane burns in oxygen to form carbon dioxide and water. CH4(9) + 2O2(g) → CO2(g) + 2H2O(1) The bond energies are shown in the table. bond bond energy in kJ/mol 410
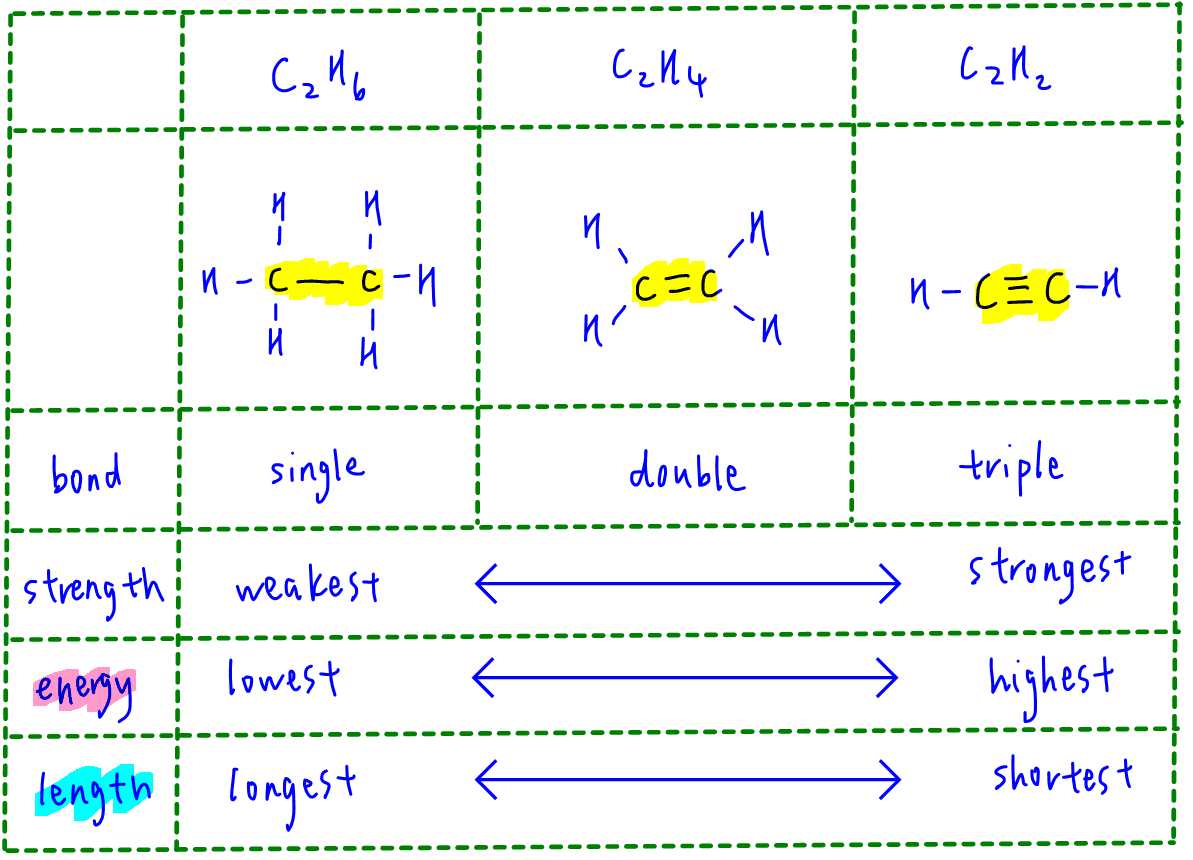
Which of the following has the strongest carbon-carbon bond ?C_{2}H_{2}C_{2}H_{4}C_{2}H_{6}C_{3}H_{8}C_{3}H_{6}

physical chemistry - Why is the bond energy for a C=O bond higher in CO2? - Chemistry Stack Exchange

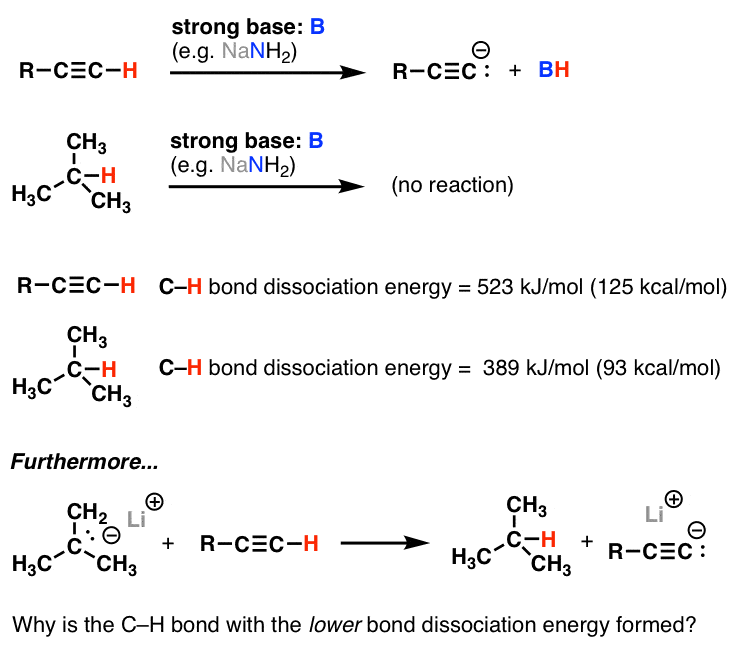
The Bond energy of C=C, C-C, C-H and H-H are 147, 83, 99 and 104 kcal/mol respectively then calculat
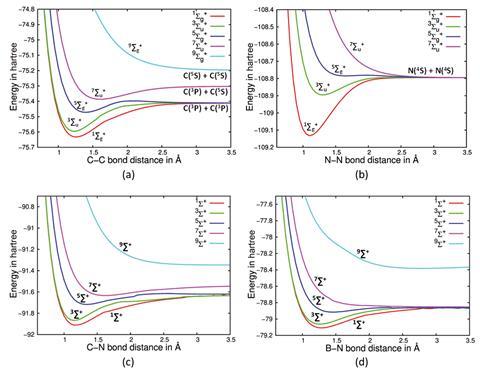
Excited state potential energy curves reignite diatomic carbon's bond order conundrum | Research | Chemistry World
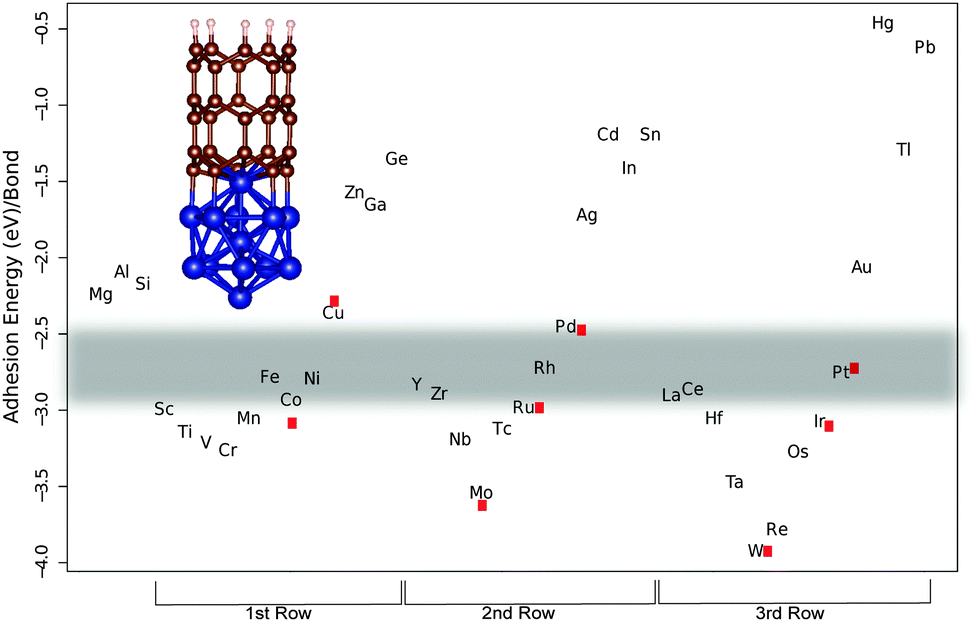
Establishing the most favorable metal–carbon bond strength for carbon nanotube catalysts - Journal of Materials Chemistry C (RSC Publishing) DOI:10.1039/C5TC00143A



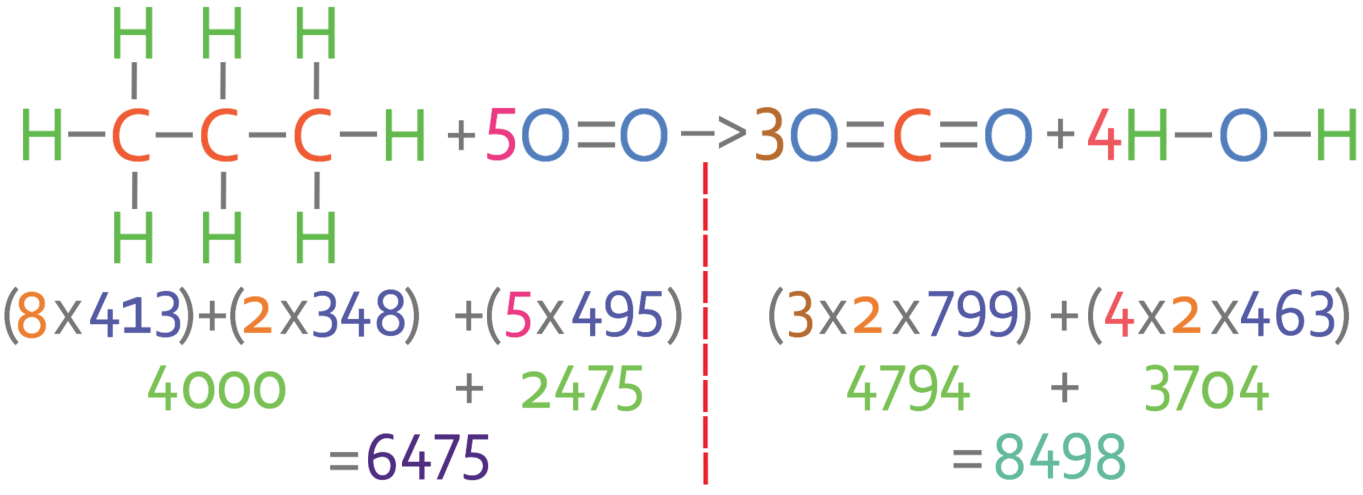


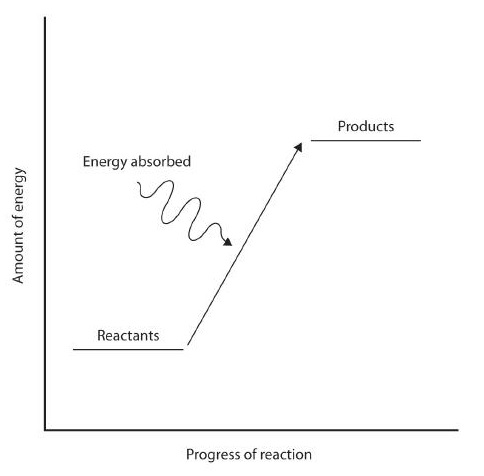.jpg?revision=1)