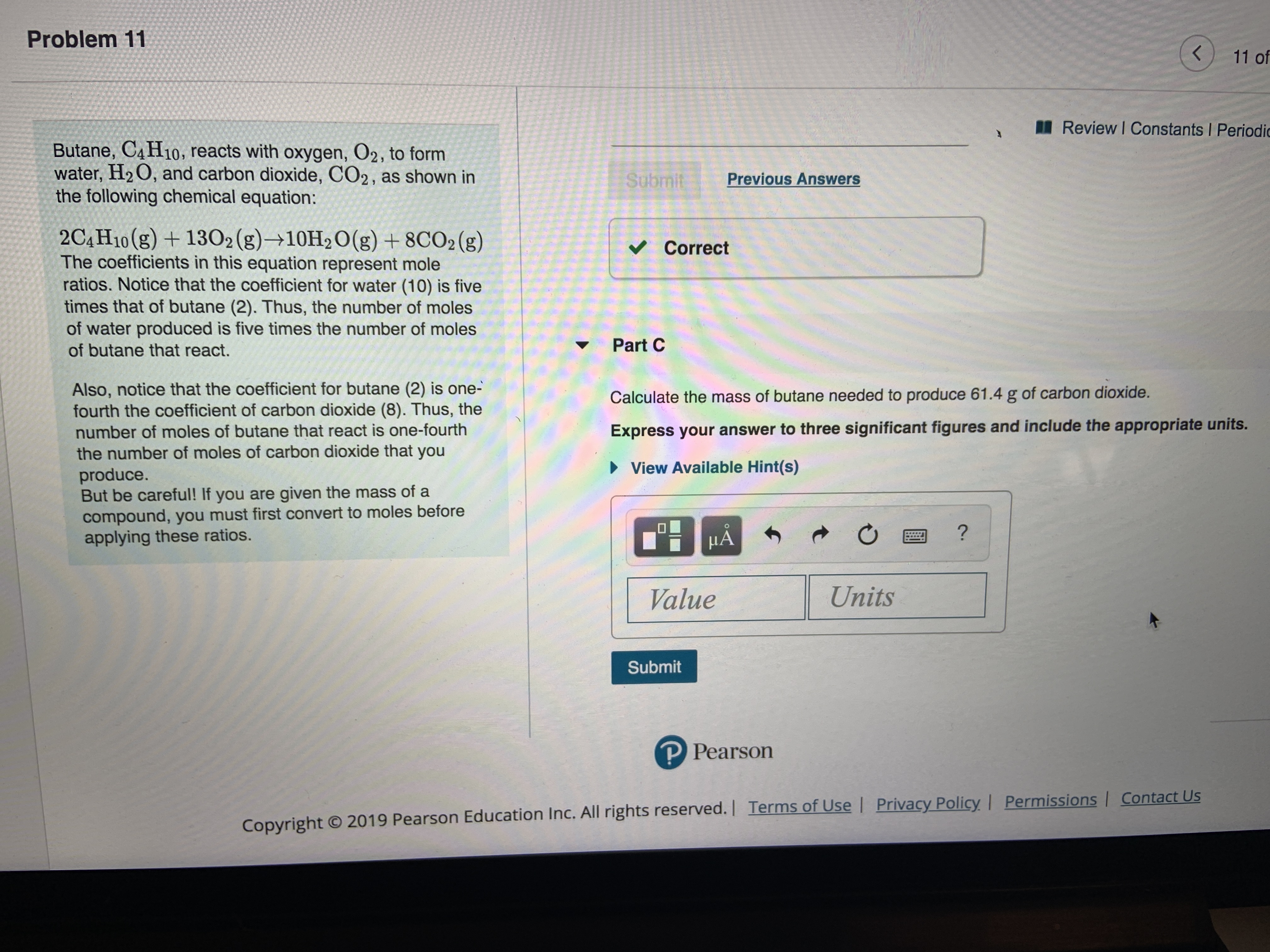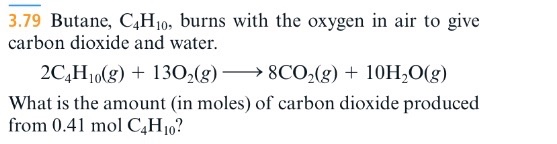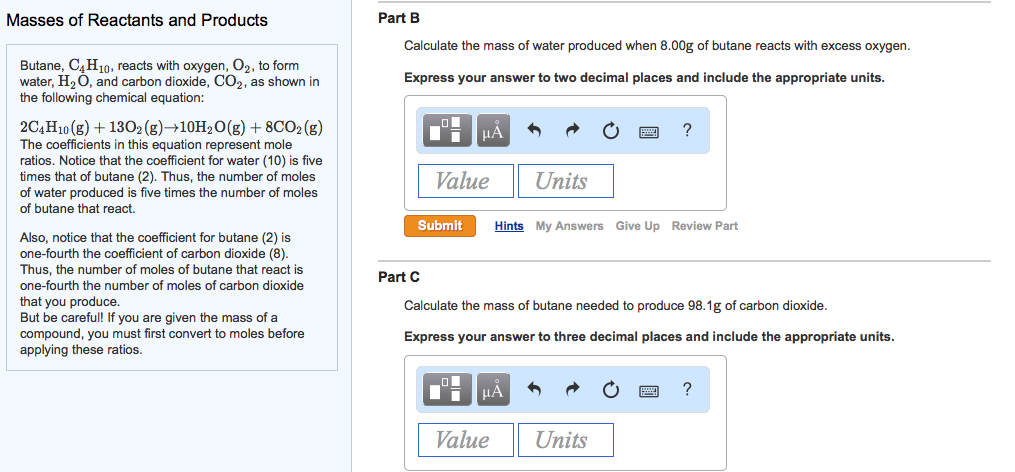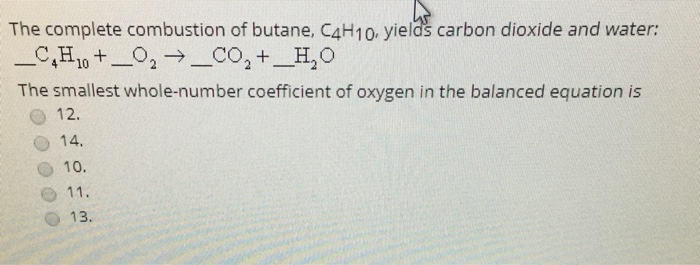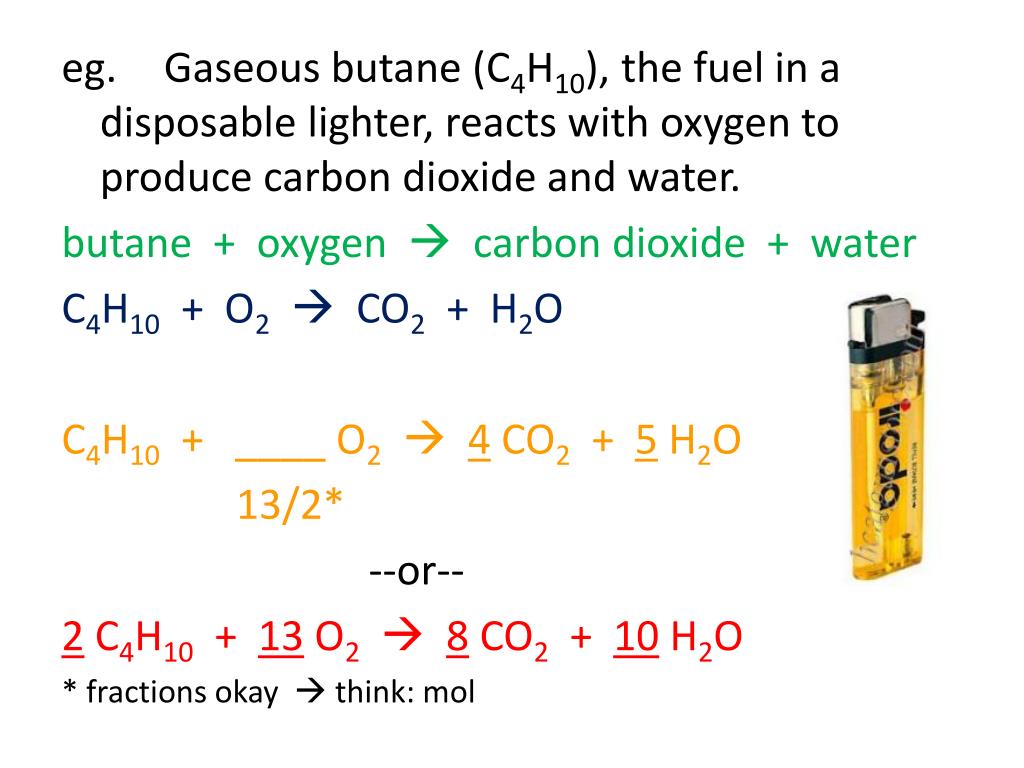
SOLVED: Gaseous butane, C4H10, reacts with diatomic oxygen gas to yield gaseouscarbon dioxide and water vapor.

When butane burns in oxygen, it produces carbon dioxide and water. This reaction is represented in - Brainly.com
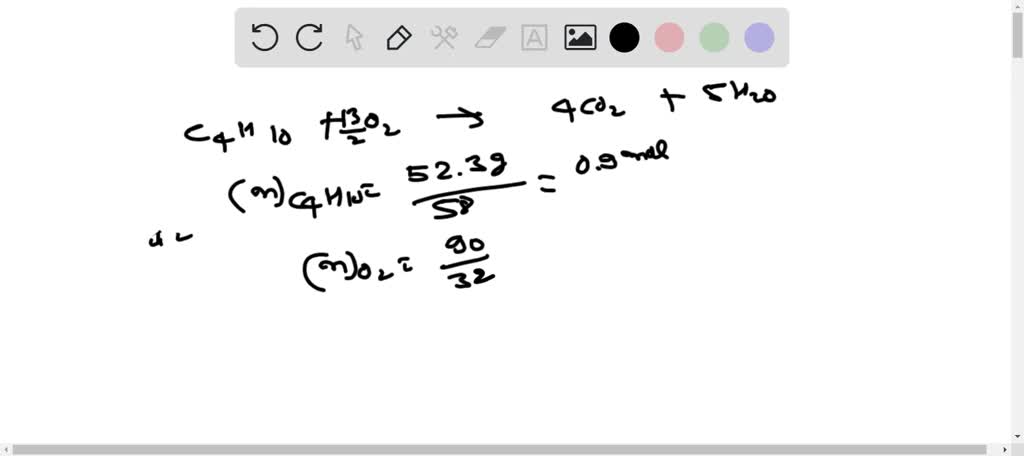
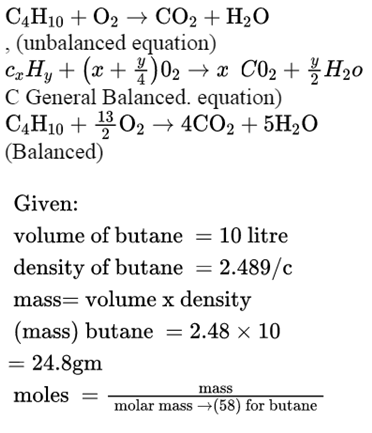
SOLVED: Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 5.23 g of butane is mixed with 9.5 g of oxygen. Calculate the maximum
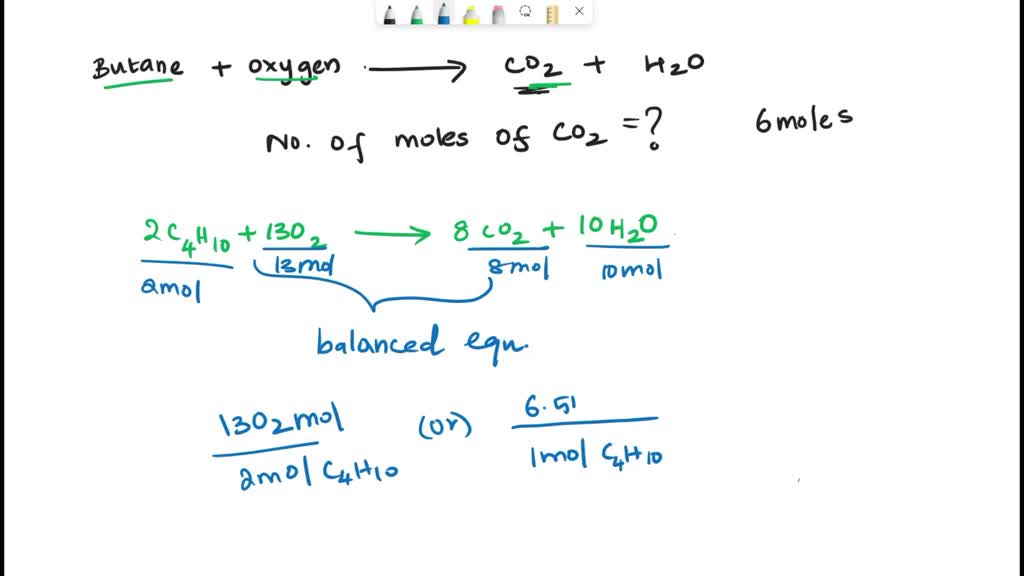
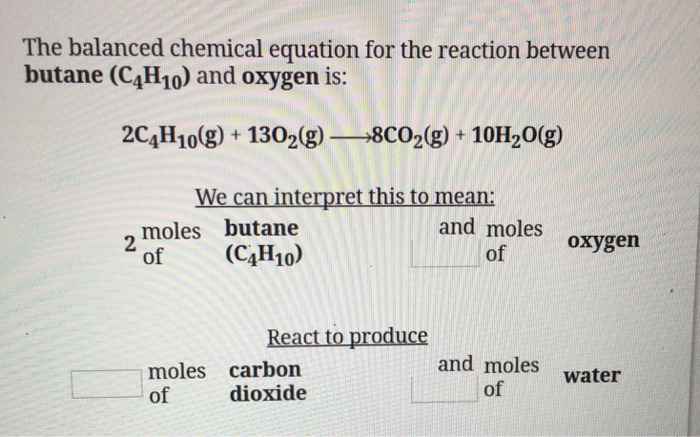
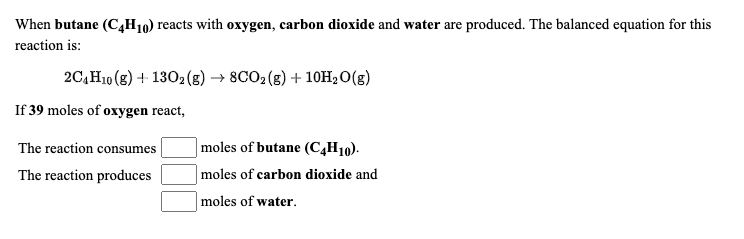
SOLVED: When butane (C4H10) reacts with oxygen, carbon dioxide and water are produced. The balanced equation for this reaction is: 2C4H10 (g) + 13O2 (g) → 8CO2 (g) + 10H2O (g) How

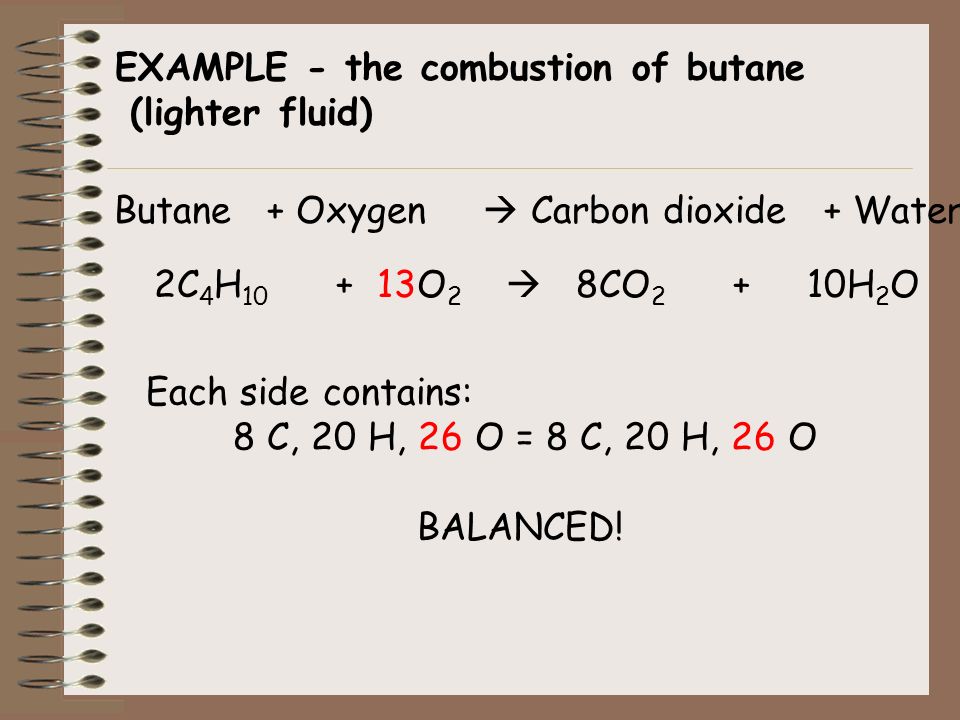
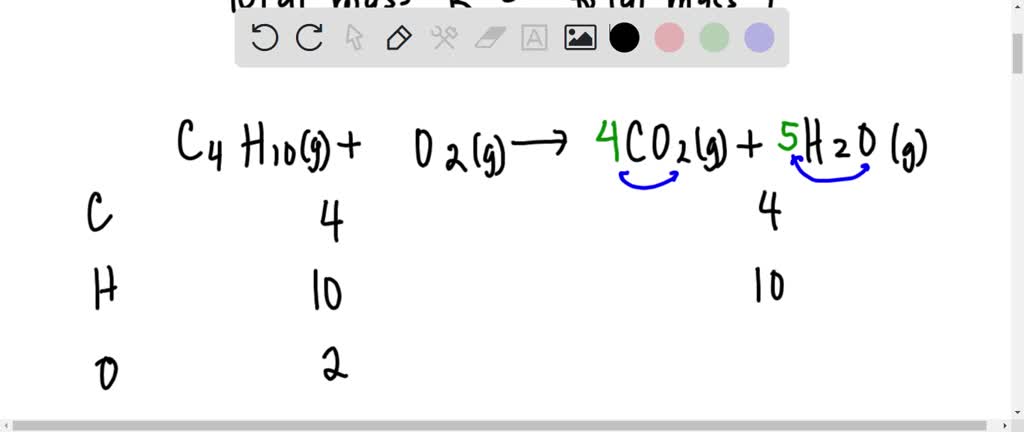
Butane (C(4)H(10)) gas burns in oxygen to give carbon dioxide and water accoding to the reaction. 2C(4)H(10) (g) + 13O(2)(g) rarr 8 CO(2)(g) + 10H(2)O(l) When 5.0 L of butane ware burnt

When 0.340 mol of butane, C_4H_{10}, are burned with excess oxygen giving CO_2 and H_2O, how many moles of oxygen are consumed? | Homework.Study.com